The New Era of Alzheimer's Diagnosis: What a FDA-Approved Blood Test Truly Means
- Research Staff

- Sep 23, 2025
- 5 min read
Updated: Sep 24, 2025

The new generation of Alzheimer's blood tests isn't based on a single breakthrough, but a culmination of decades of research.

The ability to diagnose Alzheimer’s disease simply and cost-effectively took a major step forward in recent years, with researchers developing tests that can detect key markers of the disease in a blood sample.
While a specific test hypothetically approved in May 2025 remains a future event, the groundwork laid by current advancements has already begun to revolutionize the field. These developments are intended for adults, typically 55 or older, who are experiencing cognitive decline.
The new generation of blood tests isn't based on a single breakthrough, but a culmination of decades of research into the toxic proteins that define Alzheimer's disease: amyloid-beta and tau. While several such tests are available today through specific laboratories, the milestone of full FDA approval for widespread diagnostic use is considered the ultimate game changer.

To understand the significance of this development and what it means for the future of Alzheimer’s diagnosis and treatment, we turned to the work and opinions of experts like Dr. Andrew Budson, a leading neurologist and lecturer at Harvard Medical School. Their insights help clarify not just the science, but the profound human impact of this diagnostic revolution.
The Science: From Spinal Taps to a Simple Blood Draw
For decades, the "gold standard" for confirming the presence of Alzheimer's pathology in a living person was through two primary methods: a positron emission tomography (PET) scan of the brain or a cerebrospinal fluid (CSF) analysis via a lumbar puncture (spinal tap).
PET Scans are highly accurate but are also incredibly expensive (often costing $5,000 or more), not covered by all insurance, and only available at specialized medical centers.
Spinal Taps are less expensive but are invasive, can be painful, and carry risks like headaches or infection, making many patients hesitant to undergo the procedure.
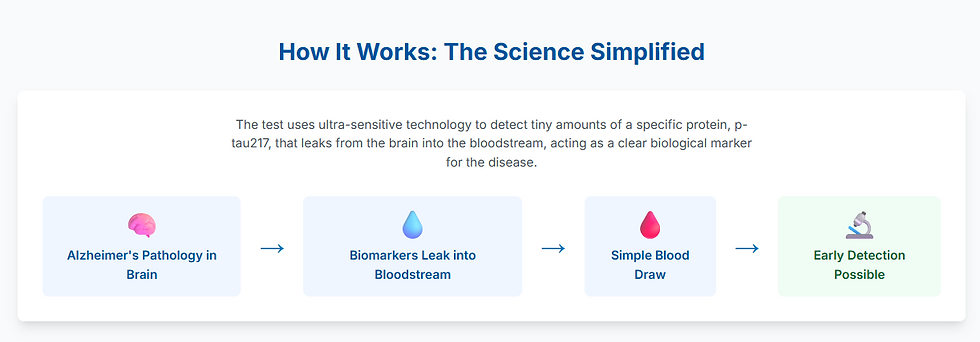
This diagnostic bottleneck has been a major barrier to early and widespread diagnosis. The new blood tests circumvent these issues entirely. They use ultra-sensitive technology to measure tiny amounts of specific Alzheimer's biomarkers that have leaked from the brain into the bloodstream. The most promising of these is phosphorylated tau-217 (p-tau217), a form of the tau protein that is a highly specific indicator of both amyloid plaques and tau tangles—the two core pathologies of Alzheimer's. Studies have shown that the accuracy of tests like these can rival that of PET scans and spinal taps.
Why a Blood Test is a Game Changer
The approval and widespread adoption of an Alzheimer's blood test will have cascading effects across medicine and society.

1. Unprecedented Accessibility: A simple, low-cost blood test can be administered in any primary care doctor's office. This democratizes diagnosis, moving it from elite neurological centers to community clinics. Patients in rural or underserved areas, who may be hours away from a facility with a PET scanner, will have access to the same level of early detection.
2. Earlier and More Accurate Diagnosis: Many individuals with mild cognitive impairment are currently diagnosed based on symptoms alone, which can be caused by a variety of conditions, from vitamin deficiencies to depression. A blood test provides objective, biological evidence, allowing doctors to distinguish Alzheimer's from other forms of dementia much earlier and with greater confidence.
3. A Gateway to New Treatments: The arrival of blood tests coincides with the approval of a new class of Alzheimer's drugs, such as lecanemab (Leqembi), which are designed to clear amyloid plaques from the brain. These treatments are most effective in the earliest stages of the disease. An accessible blood test is the essential first step to identifying the right patients who can benefit from these therapies before significant and irreversible brain damage has occurred.
4. Revolutionizing Research: The ability to quickly, cheaply, and non-invasively screen thousands of people will accelerate clinical trials for the next generation of Alzheimer's drugs. Researchers will be able to identify at-risk individuals and track the biological effects of new therapies more effectively.
An Expert's Perspective: Promise and Prudence
As Dr. Andrew Budson and other experts in the field have noted, while the enthusiasm for these tests is warranted, it must be paired with caution and careful implementation. The primary challenge is ensuring that clinicians and patients understand what the test results mean.

A "positive" result on a blood test is not a diagnosis of dementia in itself, especially in a person with no symptoms. It is an indicator of underlying brain pathology. This creates new ethical and counseling challenges. How do you tell a healthy 60-year-old that they have the building blocks of Alzheimer's in their brain? It underscores the need for robust patient education and counseling to manage the psychological impact of such knowledge.
Furthermore, physicians, particularly in primary care, will need training on how to interpret the results, when to order confirmatory tests, and how to guide patients through the complex decisions that follow, including lifestyle interventions and potential enrollment in new treatments.
The Road Ahead
While the hypothetical approval of a test like "Lumipulse" in 2025 highlights a future we are rapidly approaching, the revolution in Alzheimer's diagnosis is already underway. The science is sound, and the early versions of these tests are already being used in clinical settings and research.
The final hurdles are regulatory and educational. Full FDA approval will standardize the process and open the door for broad insurance coverage. Just as importantly, educating doctors and the public on the power and limitations of these tools will be crucial.
The era of waiting for definitive symptoms while the brain deteriorates is closing. We are entering a new age of biological insight, where a simple blood test can provide a window into the brain, offering the clarity and foresight needed to finally change the course of Alzheimer's disease.
Sources
Food and Drug Administration. (2025, May 15). FDA clears first blood test used in diagnosing Alzheimer's disease. https://www.fda.gov/news-events/press-announcements/fda-clears-first-blood-test-used-diagnosing-alzheimers-disease
Mayo Clinic. (2025, July 7). New FDA-approved blood tests for diagnosing Alzheimer's disease. https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/in-depth/new-blood-tests-alzheimers/art-20585060
Harvard Health Publishing. (2025, August 31). The new Alzheimer's blood test: What it means for diagnosis. https://www.health.harvard.edu/mind-and-mood/the-new-alzheimers-blood-test-what-it-means-for-diagnosis
Alzheimer's Association. (2025, July 28). New clinical practice guideline for blood-based biomarkers. https://aaic.alz.org/releases-2025/clinical-practice-guideline-blood-based-biomarkers.asp
Fisher Center for Alzheimer’s Research Foundation. (2025, May 27). What to know about the new blood test for Alzheimer's. https://www.alzinfo.org/articles/diagnosis/what-to-know-about-the-new-blood-test-for-alzheimers/
Washington University School of Medicine. (2025, March 30). Highly accurate blood test diagnoses Alzheimer's disease, measures extent of dementia. https://medicine.washu.edu/news/highly-accurate-blood-test-diagnoses-alzheimers-disease-measures-extent-of-dementia/
Alzheimer’s Association. (2024, July 27). Alzheimer’s blood tests could improve diagnosis. https://aaic.alz.org/releases-2024/blood-tests-alzheimers-biomarkers.asp
University of Wisconsin–Madison. (2025, May 27). WRAP research helps launch first FDA-cleared blood test for Alzheimer’s. https://wrap.wisc.edu/2025/05/28/uw-madison-research-helps-launch-first-fda-cleared-blood-test-for-alzheimers/
Alzheimer’s Association. (2025, August 21). First blood test used in diagnosing Alzheimer’s cleared by FDA. https://www.alz.org/news/2025/fda-clears-blood-test-alzheimers-diagnosis
BBC News. (2025, September 9). Alzheimer’s blood test could ‘revolutionise’ diagnosis. https://www.bbc.com/news/articles/cm2ze84e8p1o
National Institutes of Health. (2025, September 17). Accurate blood test for Alzheimer’s disease. https://www.nih.gov/news-events/nih-research-matters/accurate-blood-test-alzheimer-s-disease
About BioLife Research Staff
Our team is dedicated to providing the latest insights and tools to support overall health and wellness. We believe in fostering a community that values knowledge, innovation, and patient-centered care. Through our work, we strive to bridge the gap between scientific advancements and practical health solutions, ensuring our readers have the resources they need to thrive.



