New Screening Methods for Cervical Cancer: From HPV Infection to Early Detection and Prevention
- Renaldo Pool, BHSc

- Sep 2, 2025
- 27 min read
Updated: Sep 3, 2025

Introduction
High-risk Human Papillomavirus (HPV) is the causative agent responsible for nearly 99% of cervical cancer development globally, although additional risk factors also contribute to its development. Cervical cancer is the fourth most common cancer seen worldwide, while also being the fourth leading cause of cancer-related mortality. As of 2022, more than 660 000 women were diagnosed with cervical cancer, with 350 000 dying from this disease (Makioka et al., 2024). Even though it poses a profound health risk for women across the world, more so in low- and middle-income countries, it is also considered the most preventable.
The following article highlights the essentiality of preventative cervical screening, explores the technicalities of HPV’s viral mechanisms and mode of action, and the progressive scientific advancements that aim to provide non-invasive, accurate, and reliable diagnoses. Through structured, triage approaches, females around the globe are empowered with the necessary information regarding vaccine and screening programs to mitigate persistent high-risk HPV infection and its potential progression to malignancy. In turn, it provides women with the tools to ensure their reproductive health is a priority.
Key Takeaways
High-risk HPV is considered the main factor contributing to the development of cervical cancer. However, other factors such as smoking, the length of contraceptive use, and being sexually active also play a role.
Various HPV viral mechanisms take center stage in integrating into the host genome, including regulating oncogenic activity, driving dysregulated proliferation, preventing tumor suppression, and bypassing the immune response.
Modern cervical screening methods are crucial for early detection and diagnosis; however, challenges affect female participation worldwide. Likewise, the implementation and uptake of HPV vaccinations face obstacles, particularly in low- and middle-income countries.
These challenges can be overcome through educational initiatives, national immunization strategies, and increased participation in regular screening protocols.
Alternative approaches to cervical screening include non-invasive techniques that focus on molecular and omics-based methods, where artificial intelligence (AI) can be integrated to ensure easier accessibility, greater accuracy and reliability, and increased screening participation while considering patient privacy.
What is HPV?
Forming part of the Papillomaviridae viral family, HPV has a double-stranded DNA structure, with more than 200 viral strains sequenced (Hu et al., 2022). It is also characterized as the most sexually transmitted virus worldwide, causing one in twenty new cancer diagnoses. HPV-associated cancers include cervical, anogenital, and oropharyngeal, with nearly 70% of cervical cancers caused by high-risk HPV strains 16 and 18 (Obanya et al., 2025b).
HPV’s Mode of Action: An In-Depth Look at Its Viral Mechanisms
HPV targets the basal cells of stratified squamous epithelium, which are found in the cervix, penis, vulva, anal region, and oropharynx. HPV penetrates these cells through micro-abrasions or trauma, revealing the basement membrane of the squamous epithelium tissue and allowing entry through the basal keratinocytes (Obanya et al., 2025b).
By binding to cell surface receptors, such as alpha integrins and laminins, HPV gains entry into epithelial tissue through endocytosis. The viral genome life cycle is transported to the nucleus, where it remains episomal DNA or integrates into the host genome, particularly in high-risk and prolonged infections. In long-read sequence studies, it was found that viral genome integration tends to disrupt viral E2 genes, which usually withhold E6/E7 expression. A loss of regulatory mechanisms causes overexpression of oncoproteins. With the integration of viral material into the host genome, chromosomal rearrangement occurs (Obanya et al., 2025b). For instance, the genome becomes unstable through translocations and circular DNA structures. In turn, it drives oncogenic behavior and prevents standard mechanisms that suppress tumor formation, speeding up cancer development (Makioka et al., 2024).

This viral mechanism, along with viral oncoproteins, translates to HPV’s carcinogenic potential. The oncogenes, E6 and E7, can alter essential tumor suppressor pathways. The role E6 plays in driving oncogenic behavior is its ability to bind to the p53 protein, causing the ubiquitin-mediated degradation using the E6-associated protein (E6AP) (Luo et al., 2024). The p53 protein is stopped from causing apoptosis or cell cycle inhibition when DNA damage occurs, resulting in abnormal or genetically unstable cells from proliferating (Makioka et al., 2024). E7, in turn, deactivates the retinoblastoma protein (pRb), becoming bound to its pocket domain, preventing E2F transcription factors from being captured (Makioka et al., 2024; Ouh et al., 2024). In the case of E2F being released, it continues the progression of the cell cycle, not performing its “checks,” leading to uncontrolled cellular division (Luo et al., 2024).
Furthermore, HPV integration into the host genome facilitates a change in cellular metabolism, where E6/E7 oncoproteins promote insulin-like growth factor 2 mRNA-binding protein (IGF2BP2), which is responsible for stabilizing the MYC mRNA (transcription factor) through N6-methyladenosine (m6a) alterations (Moa et al., 2023; Obanya et al., 2025b). It upregulates glycolytic enzyme expression, for example, hexokinase 2, which is responsible for the first part of the glycolytic process. Through this process, aerobic glycolysis (also known as the Warburg effect) ensues, whereby glucose uptake is elevated, increasing lactate production, as well as ATP synthesis in the presence of oxygen, ultimately adding to the proliferation of tumor cells and metastasis (Moa et al., 2023). This mechanism was demonstrated through experimental knockdown of the E6/E7 proteins or IGF2BP2 in cervical cancer cells, which decreases glycolysis, and in turn, cellular proliferation, and cancer cell metastasis (Hu et al., 2022; Obanya et al., 2025b). The opposite is true when IGF2BP2 is upregulated, implying its core role in the glycolytic pathway (Hu et al., 2022).
In addition, HPV’s capabilities extend to disrupting the DNA damage repair process by altering different pathways, which contribute to viral replication, persistent infections, and ultimately carcinogenesis (Obanya et al., 2025b). With prospective ongoing research, the various pathways involved in driving cervical cancer development can be transformed into targeted therapeutic approaches, either for current therapies available or by developing alternative RNA-based therapies (Mao et al., 2023; Obanya et al., 2025b).
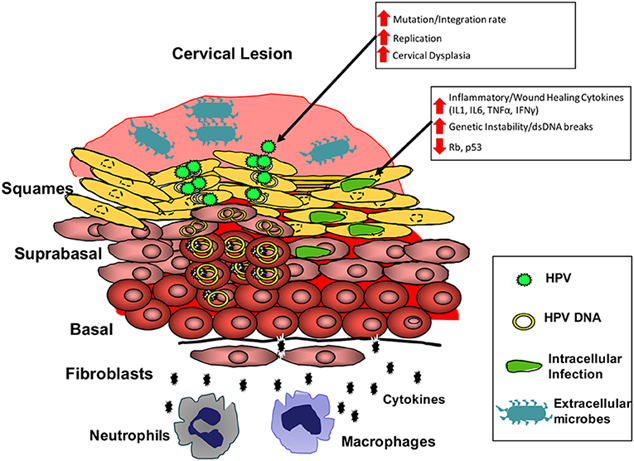
Through normal immune responses, HPV infections are mitigated and resolved within a two-year period. Recent studies indicated the significance of gamma-delta T-cells in the adaptive immune response and elevated CXCL10 chemokine concentrations from the innate immune response, which are measurable during infection clearance (Tessandier et al., 2025).
Nevertheless, how does HPV remain undetected by the immune system, you may ask? This virus evades immune detection by limiting its viral gene expression in basal cells, breaking down MHC-I, dysregulating the ability of CD8+ T-cells to recognize virally infected cells, and decreasing interferon response through various signaling pathways (Nikmanesh et al., 2025; Tessandier et al., 2025). Continuous infection leads to the accumulation of epigenetic modifications, independently of mutational changes occurring to DNA sequences, adding to an increased risk for cancer development in the form of pre-cancerous lesions and ultimately, progression to invasive carcinoma (Nikmanesh et al., 2025).
HPV and Its Transmission Routes
HPV is mainly transmitted between individuals through direct skin-to-skin contact, albeit during vaginal, anal, or oral sex. Genital-to-genital contact is the most common transmission route (Makioka et al., 2024). However, hand-to-genital contact is also a viable method of transmission (De Oliveira et al., 2025).
Alternatively, a mother can transmit the virus to her newborn during childbirth, and fomite transmission that occurs through contaminated surfaces is considered rare but possible, particularly for HPV types associated with wart formation (De Oliveira et al., 2025).
Defining Stages of Cervical Cancer
Immunohistochemical and molecular markers support the cervical intraepithelial neoplasia (CIN) grading system, aiding clinical decision-making regarding preventative measures prior to the development of invasive cervical cancer. The grading basis depends on the cervical epithelia’s abnormal cell changes and the extent of these changes (Duppala et al., 2022).
CIN1 (low-grade lesion) is described as mild dysplasia affecting the lower one-third of epithelial thickness. Low-grade lesions are more often associated with HPV infections that regress by themselves, without therapeutic intervention.
CIN2 is categorized as moderate dysplasia with abnormal cells extending into the middle third of the epithelial cells. CIN2 classification is also known to have an increased risk of cancer progression if left untreated.
CIN3, known to have higher-grade lesions, is associated with severe dysplastic cellular changes or invasive carcinoma. Abnormal cell changes affect at least two-thirds of epithelial cells, potentially the entire cellular thickness. CIN3 is also considered a precursor to invasive cervical cancer.
In invasive cancer (or carcinoma in situ), malignant cells penetrate the basement membrane of epithelial tissue, invading the underlying stroma.
Current Cervical Screening Methods
Regular cervical screening is essential for knowing your reproductive health status. It aids in the detection of precancerous or cancerous lesions in cervical cells, which are mainly attributed to high-risk HPV strain infections (Ouh et al., 2024). Identifying abnormalities in the cervical cell structures aims to treat precancerous alterations of these cells before cancer development (Liu et al., 2024; Wang et al., 2024). If cancerous cells are present, effective treatment options are investigated at an early stage of diagnosis. The different screening techniques are described in the following section.
HPV PCR Testing
Testing for high-risk types of HPV through polymerase chain reaction (PCR) is considered more effective and thus regarded as a primary screening tool in numerous countries (Xue et al., 2025). However, co-testing alongside cytological examination is also considered an effective strategy for cervical screening, preventing unnecessary follow-up testing.
Using PCR methodology enables the detection of different HPV genotypes or strains (Moberg et al., 2003). PCR is noted to have higher sensitivity than cytological examination, especially for detecting a specific HPV type, deducing that precancerous lesions could be present; this provides the necessary information that warrants further testing (Xue et al., 2025). Real-time PCR can measure the viral load in the sample, where a higher viral load is linked to an increased risk of cancer development. Follow-up testing should be considered, especially with positive test results, such as referring a patient for colposcopy or biopsy examination, which forms part of the diagnostic strategy (Devi & Podder, 2025).
Applications for Visual Inspection
Applying acetic acid or Lugol’s iodine to the cervix to visually examine precancerous or cancerous lesions is an option utilized in low-resource and technically constrained environments (Yeates et al., 2020). It provides instant results because of the color change, usually white, that occurs in the presence of abnormalities. Nowadays, integrating this technique with AI-smartphones and smartphone imaging ensures real-time evaluation by a clinician, prompt diagnostic follow-up, and further testing if necessary, promoting patient participation (Yeates et al., 2020).
Cytology Examination: Liquid-Based Cytology
Cytological examination of cervical samples involves a clinician collecting a cervical sample with a brush, which is placed in a container with liquid medium to preserve the integrity of the cells (Depuydt et al., 2003). Thereafter, a thin layer is deposited on a microscope glass slide. If present, this slide containing cervical cells is stained to enhance specific structures and highlight cell abnormalities. This process, called liquid-based cytology (LBC), is an improved technique that enhances sample and cellular quality compared to conventional Papanicolaou (Pap) smears (Depuydt et al., 2003; Thakur et al., 2024). It decreases blood, mucus, and inflammatory processes that can hinder examining cervical cells, notably seen in traditional Pap smears. Furthermore, the same sample collected for LBC examination can be used for adjunct HPV PCR testing (Thakur et al., 2024).

Immunocytochemical Staining
Once a cervical sample is collected, it can be used for immunocytochemical staining to detect p16 protein and Ki-67 (a proliferation marker) through a dual-staining approach, which is considered more effective as it reduces false negatives (Burdier et al., 2024; Luo et al., 2024; Ouh et al., 2024). These two markers indicate cells transforming due to HPV-induced activity, specifically high-risk HPV oncogenic strains. During immunocytochemical staining, the specimen is treated with antibodies against p16 and Ki-67, which produce a brown coloration of the cell cytoplasm and a red coloration of the nucleus, respectively (Wentzensen et al., 2021).
Cervical Screening Frequency
The recommended cervical screening for each age group depends on various factors, and other exceptions need to be considered, e.g., previous cervical cancer diagnosis (Abbas et al., 2023; Arrossi et al., 2024; Cervical Cancer Screening, 2025).
Age group | Recommended Cytology Screening Interval | Recommended HPV Testing Period |
21 – 29 years | Cytology examination every three years. | Not recommended for routine testing, unless cytological analysis yields abnormal results. |
30 – 65 years | Cytology screening every three years | HPV DNA testing should be considered every five years, alternatively co-testing every five years |
>65 years | In the case of previous screening results yielding an acceptable number of negative results, screening participation can be discontinued; however, if a history of persistent cervical pre-cancer or cancer or inadequate past screening exists, it is recommended that screening practices should continue. | N/A |
Table 1: The Recommended Cytology and HPV DNA testing frequency specific to each age group
Factors Impacting Cervical Screening in Women
A stigma exists where females fear and have an embarrassment associated with cervical examinations at a gynecological practice, due to misconceptions such as pain experienced during examination, removal of the reproductive system, or fear of the outcome of test results (De Oliveira et al., 2025; Gezels et al., 2025). In addition, specific communities or population groups do not believe in discussing reproductive health based on cultural beliefs, which leads to a lack of knowledge and understanding, decreasing participation in regular screening (Zheng et al., 2019; Ferguson et al., 2024; Makioka et al., 2024).
The costs associated with cervical screening are another factor reducing female participation, as seen with follow-up procedures. Where a lack of funding, insurance, or public health coverage exists, it limits accessibility of the general population to get tested (Ferguson et al., 2024).
Individuals residing in remote areas face the obstacles of limited resources, including transportation or screening centers for testing, and even a lack of healthcare services and professionals (Zheng et al., 2019; Yeates et al., 2020; Liu et al., 2024).
Lack of knowledge and low health literacy reduce awareness and prevent culturally appropriate information from circulating to the female population, discouraging females from being screened regularly (Ferguson et al., 2024; Makioka et al., 2024; De Oliveira et al., 2025).
A lack of organized screening programs also impacts the culture of efficient population screening in those areas (Liu et al., 2024).
However, health-related promotion and awareness campaigns, such as Brazil’s mobile application Se Cuida Mulher, provide educational content about HPV, indicate available health units and vaccination sites on interactive maps, and send push notifications as cervical screening reminders (De Oliveira et al., 2025). Countries can use various digital options to enhance female screening participation in this manner.
The HPV Vaccine: Development, Coverage, and Current Challenges
The WHO aims to achieve 90% vaccine coverage for girls by the age of 15 and implement 70% screening strategies, with high-performance detection methods, for females aged 35 to 45 to promote worldwide coverage and eradication of high-risk HPV infection by 2030 (Yeates et al., 2020; Rosário et al., 2022; Liu et al., 2024). Although HPV vaccination programs are well implemented in the majority of higher-income countries, low- and middle-income countries face their challenges regarding HPV vaccination availability, regardless of its cost-effectiveness and benefits.
According to 2022 statistics, 27.6% of low-income, 43.1% of low- and middle-income, and 63.9% of upper-middle-income countries have implemented vaccination strategies either on a regional or national level (Guillaume et al., 2024)
Challenges Faced
Low- and middle-income regions struggle with vaccine availability due to immunization programs not being implemented; reasons include other health concerns taking priority, a lack of healthcare resources, and logistical barriers (Kaur et al., 2023; Guillaume et al., 2024).
Financial barriers also prevent countries from rolling out vaccination programs in certain instances. However, affordable single-dose vaccinations are now being implemented, which promotes coverage in underserved countries (Kaur et al., 2023; Guillaume et al., 2024).
A general exclusion of information related to HPV screening, its transmission, recurrent infection outcomes, and progression to cervical cancer also hinders vaccine uptake (Kaur et al., 2023; De Oliveira et al., 2025). This is noticeable in conjunction with the hesitancy experienced by females due to factors such as cultural beliefs and personal privacy (Kaur et al., 2023; Guillaume et al., 2024).
One measure taken to promote vaccination uptake is educational sessions provided in schools for preteen boys and girls. These sessions are seen to have a positive influence and boost overall acceptance in communities. They also focus on inclusion and equity while increasing vaccination coverage (De Oliveira et al., 2025).
HPV Vaccine Development
As in-depthly discussed by Hoes et al. (2021), these vaccines are developed using virus-like particles (VLPs), consisting of the L1 capsid protein. They can self-assemble, forming non-infectious structures that enact the activity and properties of native virions (Hoes et al., 2021; Nikmanesh et al., 2025). Even though the vaccine constituents lack viral DNA, they promote the release of antibodies that neutralize the HPV infection (Hoes et al., 2021). After vaccination, the antibodies released by corresponding immune cells block HPV virions from attaching to the basement membrane of epithelial cell surfaces, preventing infection in the earliest stages possible (Hoes et al., 2021).
The different types of vaccines available include:
Bivalent (protects against HPV strains 16 and 18)
Quadrivalent (offers protection against HPV 6, 11, 16, and 18)
Nonavalent (protects against HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58)
How Effective is the HPV Vaccine and What is its Long-Term Impact?
Rajaram et al. (2023) elucidated on the protective efficacy of the nonavalent vaccine, indicating a 97% and 81% efficacy against high-risk disease-causing HPV in HPV-naïve females and the general population, respectively. The nonavalent vaccine also provides additional protection to seven high-risk strains of the virus. Furthermore, the bivalent vaccine indicated a 60-80% efficient protection against related strain types, such as HPV 31, 33, and 45, based on antibody cross-reactivity (Palmer et al., 2024). It was also noted during immunogenicity studies that a single dose of the HPV vaccine provided sufficient immune protection stimulated by the immune system response, equivalent to a multi-dose vaccination for persistent HPV infections (Rajaram et al., 2023).
The durability of vaccines has also indicated that protection is viable for ³10 years after following the complete vaccination program, with minimal evidence of immunity affected (Hoes et al., 2021); however, the age at which females are vaccinated, vaccine dosage, and vaccine uptake remain areas necessitating further investigation (Hoes et al., 2021). This also addresses the potential of herd immunity, whereby if more than 70% of a population is vaccinated, HPV prevalence becomes reduced even in the unvaccinated population (Abbas et al., 2023).
During a longitudinal study of vaccination uptake in Scotland, researchers have reported reduced rates of high-grade cervical dysplasia, primarily noticeable in the younger age groups receiving the vaccine since 2008 (Palmer et al., 2024). However, continuous monitoring and routine cervical screening are advised for females who have not completed the full immunization protocol (received only one or two doses), as the immunogenicity for fewer dosages is unknown (Palmer et al., 2024).
As of 2021, statistics indicate that more than 85% of high-income countries have consistent HPV vaccination programs as part of their national immunization strategy, and the nonavalent vaccine is widely implemented for broader protection (Hoes et al., 2021; Rosário et al., 2022; Guillaume et al., 2024). Continuous monitoring of vaccination efficacy is conducted through systematic reviews, randomized controlled trials, and observational studies alongside WHO guidance (Hoes et al., 2021; Rosário et al., 2022; Palmer et al., 2024). Moreover, strategic planning is continuously done to improve vaccine access, especially in low- and middle-income countries.
Addressing Vaccine Misconceptions
When it comes to participation in vaccination programs, a lack of sufficient knowledge and generalized misconceptions create ambiguity, resulting in a decreased willingness to partake (Kaur et al., 2023). Educating and having open discussions with clinicians, other female family members, and in schools increases awareness of HPV-associated risks in the unvaccinated population (De Oliveira et al., 2025).
One contributing factor to reduced vaccination uptake is the general concern regarding vaccine safety due to potential side effects or the potential to cause long-term adverse events, which is evident in specific regions and population groups (Rajaram et al., 2023; Guillaume et al., 2024). Conversely, through research conducted and global surveillance, the benefits of HPV vaccinations and the safety associated with their use have been established. Side effects are limited to mild and short reactions, for instance, tenderness at the injection site, swelling, or a mild fever (Rajaram et al., 2023).
Employing educational strategies to promote vaccination uptake raises awareness of HPV-associated cervical cancer, improves knowledge, discredits myths, and increases vaccine participation in adolescents and women (Kaur et al., 2023; De Oliveira et al., 2025). When reliable information is shared, backed by sufficient scientific evidence regarding the safety and efficacy of the HPV vaccine, it creates trustworthiness and willingness from the patient’s perspective (Guillaume et al., 2024).

Future of Cervical Screening: Exploring Alternatives to Non-Invasive Approaches for Prevention
The future of cervical screening involves investigating approaches that are considered noninvasive, molecular-based, and the integration of AI assistance for improved accuracy and reliability (Daponte et al., 2021; Alum, 2025). Katheryn Pence also explores this topic in her article regarding the usage of FemTech. As omics-focused research advances, the onset of novel biomarker discovery and application highlights the forward progression of cervical screening. The impact of utilizing different biomarkers for cervical cancer detection underscores the non-invasive nature that replaces current invasive procedures.
Biological samples such as serum, urine, menstrual blood, and even self-collected samples can be used for HPV DNA detection and cervical cancer-linked biomarkers, accentuating the need for highly sensitive and specific methods. The focal point is to promote participation in patient screening and improve accessibility to screening alongside rapid screening techniques while considering patient privacy and comfort. Consequently, this benefit provides faster referral for additional testing if needed, and earlier diagnosis leads to immediate intervention compared to conventional methods.
Using Transcriptomics to Measure Biomarkers
Biomarker measurement allows for molecular change monitoring present in blood and urine specimens. Some biomarkers include SSG-Ag, zyxin, CA-125, circulating free DNA (cfDNA), and urine non-coding RNAs such as miRNAs (e.g., miRNA-34a-5p, MMRN1, and LRG1), which show both high sensitivity and specificity for early cervical cancer detection and screening based on specific signatures produced related to cervical cancer (Ning et al., 2021; Devi & Podder, 2025). Moreover, exosomal miR-30d-5p and let-7d-3p have shown high diagnostic marker value by differentiating CIN II+ from low-grade or healthy instances. Additional circulating miRNAs have also been validated as diagnostically valuable for cervical lesion classification (Zheng et al., 2019). Small non-coding RNA (sncRNAs) studies revealed their potential as biomarkers due to their presence between benign, precancerous, and cancerous cervical tissues (Jafari et al., 2024).
These transcriptomic approaches can indicate tumor activity without the need for invasive techniques (Devi & Podder, 2025). The significance of high sensitivity and specificity implies that false negative results occur less than with current cytology methods used today (Jafari et al., 2024). It also enables earlier detection due to the presence of molecular changes before visible pre-cancerous or cancerous lesions develop (Devi & Podder, 2025). Furthermore, Makioka et al. (2024) showed that ultrasensitive enzyme-linked immunosorbent assay (ELISA) testing can detect HPV16 and 18’s E7 oncoprotein in urine. This was found to correlate with various CIN stages, providing information on the active state of oncogenic activity (Makioka et al., 2024); however, prospective investigation is necessary to substantiate this method for future clinical use.
Genomic Studies on DNA Methylation and Other DNA Biomarkers
Furthermore, genomic approaches turn to high-throughput sequencing of genetic mutations. In these sites, HPV attaches and integrates into the host genome, which can lead to DNA hypermethylation, silencing tumor suppressor genes, or hypomethylation, promoting the functioning of oncogenes (Ouh et al., 2024; Devi & Podder, 2025). DNA methylation, associated with the severity of cervical cancer seen with CIN2 and CIN3, provides a clinical picture for risk assessment and triaging purposes, through testing for FAM19A4/miR124-2 as noted in the VALID-SCREEN study conducted in the European Union (Burdier et al., 2024; Luo et al., 2024; Ouh et al., 2024).
Using genomic biomarkers present in blood, such as circulating tumor DNA (ctDNA) or circulating cell-free DNA (ccfDNA), measured by liquid biopsies – for instance, droplet digital PCR (ddPCR) – provides earlier detection of low quantities of HPV DNA present. In turn, it can serve as a monitoring tool during treatment and as a prognostic marker (Gupta et al., 2025). Identification of PRR11, through PCR techniques, serves as a genetic marker known to increase in the presence of cervical cancer, adding diagnostic and prognostic value to the screening process (Tadlaoui et al., 2024).
Metabolomics and Proteomics for Biomarker Detection in Cervical Cancer
Metabolomic and proteomic studies highlight how protein biomarker detection through mass spectrometry profiling in various samples, such as serum, cervical fluid, and urine, can differentiate cancer-associated activity from healthy outcomes (Daponte et al., 2021; Jafari et al., 2024; Devi & Podder, 2025). When changes occur in serum or urine, mass spectrometry can also show the presence of cancer-linked metabolites (Jafari et al., 2024).
Integrating Multi-Omics and AI
Ultimately, combining AI and deep learning models with multi-omics methods enhances earlier cancer detection and improves prognoses through multiple biomarker identification and evaluation, with high diagnostic accuracy and specificity (Jafari et al., 2024; Wang et al., 2024; Alum, 2025).
Lan et al. (2024) focused on applying an innovative multi-omics method integrating data (DeepKEGG) to assess the biological correlation between gene expression, miRNAs, and different signaling pathways associated with different cancers. Their model was used to predict cancer recurrence and identify cancer-specific biomarkers; despite not focusing specifically on cervical cancer, it could benefit prospective research (Lan et al., 2024). In addition to biomarker discovery, deep learning models can provide a personalized approach to diagnosis, treatment, and monitoring to benefit each patient’s outcome (Alum, 2025; Devi & Podder, 2025).
AI-Driven Cytological Screening and More
AI systems implemented in cytology analysis can analyze cytology slides and capture images of various cell types. In this manner, they aid in identifying abnormalities faster than manual slide review, simultaneously decreasing the risk of human error (Wang et al., 2024). Integrating AI and machine learning models with cytological examination provides a higher sensitivity, e.g., 94%, for high-grade squamous intraepithelial lesion (HSIL) cases. The Artificial Intelligence Cervical Cancer Screening (AICCS) system accurately classifies cervical cells based on a diverse dataset training. At the same time, studies have indicated highly accurate grading, with a sensitivity of 94.6% and specificity of 89%, which performs better than the manual slide reviews by pathologists (Wang et al., 2024).
An alternative approach to the conventional acetic acid method includes remote experts (healthcare practitioners and clinicians) utilizing secure smartphone-based screening techniques in Tanzania to inspect cervical images in real time (Yeates et al., 2020). This is seen with their SEVIA program, which improved diagnostic accuracy by 32% in low-resource environments (Ferguson et al., 2024). During this screening technique, 3-5% acetic acid is applied to the cervix, highlighting precancerous or cancerous areas if present, and in-app images are shared with professionals, providing guidance, diagnostic expertise, and treatment options, if applicable (Yeates et al., 2020; Ferguson et al., 2024). Furthermore, training AI models on smartphone-based images with acetic acid application highlights a 94% accuracy in precancerous lesion detection, lessening specialized technical expert analysis, especially in low-resource settings experiencing various limitations relating to cervical screening (Ferguson et al., 2024).
However, existing challenges with AI-assistance integration should be addressed to enhance machine learning and deep learning models for implementation in clinical settings (Alum, 2025). For instance, different machine learning approaches should be compared to enhance population diversity datasets to improve generalizability and AI-combined cervical screening. Deep learning techniques should be implemented for intricate datasets, e.g., atypical glandular cells associated with adenocarcinoma, for trend analyses and predictive capabilities that small-scale models could miss (Alum, 2025; Xue et al., 2025).
By assessing a wide range of cytology and biomarker samples in real time with AI models, each patient’s case can be reviewed for cancer-driven cell presence or pre-cancerous alterations. This enables faster diagnoses with immediate intervention approaches and, in other cases, could enhance preventative techniques and risk detection (Alum, 2025; Jafari et al., 2024; Xue et al., 2025).
Ultimately, AI can be used as an automated triage system, determining which patients require additional diagnostic testing through colposcopy referral or whether an immediate treatment regimen is required (Wang et al., 2024; Xue et al., 2025). It allocates resources for specific circumstances and prevents patient intervention delays when needed. With AI integration, it strengthens the WHO’s targets through additional efforts to increase female routine screening participation. AI implementation also presents innovative solutions to enhancing routine screening, especially in remote or underserved regions, offering a promising outlook for the global future of cervical screening access.
The Future of HPV DNA Testing: Self-Collection Samples
An alternative approach to HPV DNA testing is incorporating adhesive strips to menstrual pads for high-risk HPV strain detection (Chakravarti et al., 2022). Research has indicated a good agreement between menstrual blood and conventional cervical sample collection for HPV testing; females are also inclined to use this preferred method due to its ease of use, with more privacy regarding sample collection (Chakravarti et al., 2022).
With patient privacy prioritized, another method implemented globally, especially in low-resource settings, is self-sampling kits for HPV DNA testing (Daponte et al., 2021; Ferguson et al., 2024; Gezels et al., 2025). Studies have also indicated close accuracy concerning clinician-collected samples compared to self-sampling (Daponte et al., 2021; Gezels et al., 2025). The idea behind emphasizing self-collection and added privacy for women is to promote screening participation, specifically in population groups where cervical screening is not actively conducted (Gezels et al., 2025). In turn, it confronts patient education gaps and logistical issues, especially in low- and middle-income regions (Ferguson et al., 2024; Gezels et al., 2025).
HPV DNA testing can also be conducted using a patient's first-void urine; the first void is preferable as it preserves DNA present, mitigating its degradation. This is also considered a more straightforward sample collection method, with added patient privacy (Daponte et al., 2021; Burdier et al., 2024).
Alternative Screening Techniques: Breathomics
Even though breathomics is a concept known for other cancer detection, for instance, in breast cancer, breath analysis of volatile organic compounds (VOCs) is an optimistic alternative to noninvasive techniques for diagnosing cancer presence (Zhou et al., 2017). This method can be altered and applied to cervical cancer screening in the future. As highlighted by Zhou et al. (2017), whereby VOCs were measured using a proton transfer reaction mass spectrometer (PTR-MS), limitations include that this study needs to be repeated in a larger population group.
Conclusion
Despite global initiatives that address the mitigation of HPV spread and persistent infections caused by high-risk genotypes, low- and middle-income countries still face challenges that demand thorough strategic planning. However, the focus remains on providing informative patient educational content, ensuring individuals from all walks of life advocate for access to screening protocols, vaccination programs, and advancing technologies, emphasizing the importance of understanding one's reproductive health status.
Technological advancements, innovative scientific research, and collaborative efforts among the research community, pharmaceutical industries, and government sectors hold a significant promise for high-income and low- and middle-income countries. With the continuous development of innovative platforms, non-invasive cervical screening methods, and the integrative power of AI and deep learning models, women worldwide will have more tools for regular, easy-to-use screening, appropriate screening intervals, accurate diagnostics, and better reproductive health management.
Finally, cervical cancer prevention and elimination remain a shared responsibility between scientific progress and global initiatives. Women everywhere can make a meaningful change today, shaping the trajectory for future generations. Use the knowledge and insights from this article to empower yourself and other women around you, because together, resilience and prevention will advance health for women everywhere.
References
1. Alum E. U. (2025). AI-driven biomarker discovery: enhancing precision in cancer diagnosis and prognosis. Discover oncology, 16(1), 313. https://doi.org/10.1007/s12672-025-02064-7
2. Arrossi, S., Straw, C., Antelo, V. S., Paolino, M., Baena, A., Forestier, M., Rol, M., & Almonte, M. (2024). Implementation of WHO guidelines for cervical cancer screening, diagnosis and treatment: knowledge and perceptions of health providers from Argentina. BMC Cancer, 24(1). https://doi.org/10.1186/s12885-024-12650-7
3. Burdier, F. R., Waheed, D. E., Nedjai, B., Steenbergen, R. D. M., Poljak, M., Baay, M., Vorsters, A., & Van Keer, S. (2024). DNA methylation as a triage tool for cervical cancer screening - A meeting report. Preventive medicine reports, 41, 102678. https://doi.org/10.1016/j.pmedr.2024.102678
4. Cervical Cancer Screening. (2025). National Cancer Institute. Retrieved June 21, 2025, from https://www.cancer.gov/types/cervical/screening
5. Chakravarti, P., Maheshwari, A., Tahlan, S., Kadam, P., Bagal, S., Gore, S., Panse, N., Deodhar, K., Chaturvedi, P., Dikshit, R., & Budukh, A. (2022). Diagnostic accuracy of menstrual blood for human papillomavirus detection in cervical cancer screening: a systematic review. Ecancermedicalscience, 16, 1427. https://doi.org/10.3332/ecancer.2022.1427
6. Daponte, A., Michail, G., Daponte, A.-I., Daponte, N., & Valasoulis, G. (2021). Urine HPV in the Context of Genital and Cervical Cancer Screening—An Update of Current Literature. Cancers, 13(7), 1640. https://doi.org/10.3390/cancers13071640
7. De Oliveira, A. S., Souza, A. C. D. S., Oliveira, B. S., & De França, L. M. C. (2025). Se Cuida Mulher: a Knowledge Dissemination App for Engagement in Public Policies for Cervical Cancer Prevention. Revista De Gestão E Secretariado (Management and Administrative Professional Review), 16(4), e4846. https://doi.org/10.7769/gesec.v16i4.4846
8. Depuydt, C. E., Vereecken, A. J., Salembier, G. M., Vanbrabant, A. S., Boels, L. A., van Herck, E., Arbyn, M., Segers, K., & Bogers, J. J. (2003). Thin-layer liquid-based cervical cytology and PCR for detecting and typing human papillomavirus DNA in Flemish women. British journal of cancer, 88(4), 560–566. https://doi.org/10.1038/sj.bjc.6600756
9. Devi, S., & Podder, L. (2025). Role of Blood and Urine-Based Novel Biomarkers in Cervical Cancer Detection, Screening, Prognosis: Current advances and future directions. Trends in Sciences, 22(5), 9522. https://doi.org/10.48048/tis.2025.9522
10. Duppala, S. K., Yadala, R., Velingkar, A., Suravajhala, P., Pawar, S. C., & Vuree, S. (2022). Integrative Multi-Omics approaches for identifying Cervical cancer therapeutic targets. bioRxiv (Cold Spring Harbor Laboratory). https://doi.org/10.1101/2022.10.07.511244
11. Ferguson, A. L., Erwin, E., Sleeth, J., Symonds, N., Chard, S., Yuma, S., Oneko, O., Macheku, G., Andrews, L., West, N., Chelva, M., Ginsburg, O., & Yeates, K. (2024). An Implementation Evaluation of the Smartphone-Enhanced Visual Inspection with Acetic Acid (SEVIA) Program for Cervical Cancer Prevention in Urban and Rural Tanzania. International journal of environmental research and public health, 21(7), 878. https://doi.org/10.3390/ijerph21070878
12. Guillaume, D., Waheed, D. E., Schleiff, M., Muralidharan, K. K., Vorsters, A., & Limaye, R. J. (2024). Global perspectives of determinants influencing HPV vaccine introduction and scale-up in low- and middle-income countries. PloS one, 19(1), e0291990. https://doi.org/10.1371/journal.pone.0291990
13. Gupta, A., Dagar, G., Das, S. K., Chauhan, R., Shankar, A., Sharma, D. N., Suri, V., Khan, M. A., Macha, M. A., Ahmed, I., Akil, A. S. A., Bhat, A. A., & Singh, M. (2025). Prognostic value of circulating HPV cell-free DNA in cervical cancer using liquid biopsy. Scientific Reports, 15(1). https://doi.org/10.1038/s41598-025-93152-9
14. Hoes, J., Pasmans, H., Schurink-van ’t Klooster, T. M., van der Klis, F. R. M., Donken, R., Berkhof, J., & de Melker, H. E. (2021). Review of long-term immunogenicity following HPV vaccination: Gaps in current knowledge. Human Vaccines & Immunotherapeutics, 18(1). https://doi.org/10.1080/21645515.2021.1908059
15. Hu, C., Liu, T., Han, C., Xuan, Y., Jiang, D., Sun, Y., Zhang, X., Zhang, W., Xu, Y., Liu, Y., Pan, J., Wang, J., Fan, J., Che, Y., Huang, Y., Zhang, J., Ding, J., Yang, S., Yang, K. (2022). HPV E6/E7 promotes aerobic glycolysis in cervical cancer by regulating IGF2BP2 to stabilize m6A-MYC expression. International Journal of Biological Sciences, 18(2), 507-521. https://doi.org/10.7150/ijbs.67770
16. Jafari, A., Farahani, M., Abdollahpour-Alitappeh, M., Manzari-Tavakoli, A., Yazdani, M., & Rezaei-Tavirani, M. (2024). Unveiling diagnostic and therapeutic strategies for cervical cancer: biomarker discovery through proteomics approaches and exploring the role of cervical cancer stem cells. Frontiers in Oncology, 13. https://doi.org/10.3389/fonc.2023.1277772
17. Kaur, S., Sharma, L. M., Mishra, V., Goyal, M. G. B., Swasti, S., Talele, A., & Parikh, P. M. (2023). Challenges in Cervical Cancer Prevention: Real-World Scenario in India. South Asian journal of cancer, 12(1), 9–16. https://doi.org/10.1055/s-0043-1764222
18. Lan, W., Liao, H., Chen, Q., Zhu, L., Pan, Y., & Chen, Y. P. (2024). DeepKEGG: a multi-omics data integration framework with biological insights for cancer recurrence prediction and biomarker discovery. Briefings in Bioinformatics, 25(3). https://doi.org/10.1093/bib/bbae185
19. Luo, H., Lian, Y., Tao, H., Zhao, Y., Wang, Z., Zhou, J., Zhang, Z., & Jiang, S. (2024). Relationship between p16/ki67 immunoscores and PAX1/ZNF582 methylation status in precancerous and cancerous cervical lesions in high-risk HPV-positive women. BMC Cancer, 24(1). https://doi.org/10.1186/s12885-024-12920-4
20. Makioka, D., Inada, M., Awano, M., Saito, E., Shinoda, T., Abe, S., Yoshimura, T., Müller, M., Sasagawa, T., & Ito, E. (2024). Quantification of HPV16 E7 Oncoproteins in Urine Specimens from Women with Cervical Intraepithelial Neoplasia. Microorganisms, 12(6), 1205. https://doi.org/10.3390/microorganisms12061205
21. Mao, Z., Wang, B., Zhang, T., & Cui, B. (2023). The roles of m6A methylation in cervical cancer: functions, molecular mechanisms, and clinical applications. Cell Death and Disease, 14(11). https://doi.org/10.1038/s41419-023-06265-2
22. Moberg, M., Gustavsson, I., & Gyllensten, U. (2003). Real-time PCR-based system for simultaneous quantification of human papillomavirus types associated with high risk of cervical cancer. Journal of clinical microbiology, 41(7), 3221–3228. https://doi.org/10.1128/JCM.41.7.3221-3228.2003
23. Nikmanesh, N., Safarpour, A. R., Niknam, R., & Nikmanesh, Y. (2025). Short communication on HPV infection, pathogenesis of cancer, immune cells involved in infection, vaccination, and suggested treatments. Journal of Cancer Immunology, 7(2), 72–80. https://doi.org/10.33696/cancerimmunol.7.106
24. Ning, R., Meng, S., Wang, L., Jia, Y., Tang, F., Sun, H., Zhang, Z., Zhang, C., Fan, X., Xiao, B., Yang, C., & Li, S. (2021). 6 Circulating miRNAs can be used as Non-invasive Biomarkers for the Detection of Cervical Lesions. Journal of Cancer, 12(17), 5106–5113. https://doi.org/10.7150/jca.51141
25. Obanya, D. I., Wootton, L. M., & Morgan, E. L. (2025b). Advances in understanding the mechanisms of the human papillomavirus oncoproteins. Biochemical Society Transactions. https://doi.org/10.1042/bst20253041
26. Palmer, T. J., Kavanagh, K., Cuschieri, K., Cameron, R., Graham, C., Wilson, A., & Roy, K. (2024). Invasive cervical cancer incidence following bivalent human papillomavirus vaccination: a population-based observational study of age at immunization, dose, and deprivation. JNCI Journal of the National Cancer Institute, 116(6), 857–865. https://doi.org/10.1093/jnci/djad263
27. Rajaram, S., Sahoo, I., Heda, A., & Singh, L. (2023). Human papillomavirus vaccine. Current Medicine Research and Practice, 13(2), 62–68. https://doi.org/10.4103/cmrp.cmrp_28_23
28. Rosário, A., Sousa, A., Marinho‐Dias, J., Medeiros, R., Lobo, C., Leça, L., Coimbra, N., Tavares, F., Baldaque, I., Martins, G., Monteiro, P., Henrique, R., & Sousa, H. (2022). Impact of high‐risk Human Papillomavirus genotyping in cervical disease in the Northern region of Portugal: Real‐world data from regional cervical cancer screening program. Journal of Medical Virology, 95(1). https://doi.org/10.1002/jmv.28414
29. Tadlaoui, K., Sosse, S., Tiabi, I., Aqerrout, M., Souiri, A., Benhessou, M., & Ennaji, M. (2024). Screening for proline‑rich protein 11 gene expression in cervical cancer: Use as a novel diagnostic biomarker and poor prognostic factor. Medicine International, 5(1). https://doi.org/10.3892/mi.2024.202
30. Tessandier, N., Elie, B., Boué, V., Selinger, C., Rahmoun, M., Bernat, C., Grasset, S., Groc, S., Bedin, A., Beneteau, T., Bonneau, M., Graf, C., Jacobs, N., Kamiya, T., Kerioui, M., Lajoie, J., Melki, I., Prétet, J., Reyné, B., . . . Alizon, S. (2025). Viral and immune dynamics of genital human papillomavirus infections in young women with high temporal resolution. PLoS Biology, 23(1), e3002949. https://doi.org/10.1371/journal.pbio.3002949
31. Thakur, N., Aryal, G., & Rana, R. (2024). Retrospective study of correlation between high risk detection of HPV and liquid based cervical cytology for cervical cancer screening. Nepal Mediciti Medical Journal, 5(2), 49–54. https://doi.org/10.3126/nmmj.v5i2.74088
32. Wang, J., Yu, Y., Tan, Y., Wan, H., Zheng, N., He, Z., Mao, L., Ren, W., Chen, K., Lin, Z., He, G., Chen, Y., Chen, R., Xu, H., Liu, K., Yao, Q., Fu, S., Song, Y., Chen, Q., . . . Yao, H. (2024). Artificial intelligence enables precision diagnosis of cervical cytology grades and cervical cancer. Nature Communications, 15(1). https://doi.org/10.1038/s41467-024-48705-3
33. Wentzensen, N., Lahrmann, B., Clarke, M. A., Kinney, W., Tokugawa, D., Poitras, N., Locke, A., Bartels, L., Krauthoff, A., Walker, J., Zuna, R., Grewal, K. K., Goldhoff, P. E., Kingery, J. D., Castle, P. E., Schiffman, M., Lorey, T. S., & Grabe, N. (2021). Accuracy and Efficiency of Deep-Learning-Based Automation of Dual Stain Cytology in Cervical Cancer Screening. Journal of the National Cancer Institute, 113(1), 72–79. https://doi.org/10.1093/jnci/djaa066
34. Xu, M. L., Kim, H. J., Kim, S. C., Ju, W., Kim, Y. H., Chang, K. H., & Kim, H. J. (2019). Serum anti-GAPDH autoantibody levels reflect the severity of cervical lesions: A potential serum biomarker for cervical cancer screening. Oncology letters, 18(1), 255–264. https://doi.org/10.3892/ol.2019.10326
35. Xue, P., Dang, L., Kong, L., Tang, H., Xu, H., Weng, H., Wang, Z., Wei, R., Xu, L., Li, H., Niu, H., Wang, M., Ye, Z., Li, Z., Chen, W., Pan, Q., Zhang, X., Rezhake, R., Zhang, L., . . . Zhao, F. (2025). Deep learning enabled liquid-based cytology model for cervical precancer and cancer detection. Nature Communications, 16(1). https://doi.org/10.1038/s41467-025-58883-3
36. Yeates, K., Erwin, E., Mtema, Z., Magoti, F., Nkumbugwa, S., Yuma, S., Hopman, W. M., Ferguson, A., Oneko, O., Macheku, G., Mtei, A. F., Smith, C., Andrews, L., West, N., Dalton, M., Newcomb, A., & Ginsburg, O. (2020). Smartphone-Enhanced Training, QA, Monitoring, and Evaluation of a Platform for Secondary Prevention of Cervical Cancer: Opportunities and Challenges to Implementation in Tanzania. JCO global oncology, 6, 1114–1123. https://doi.org/10.1200/GO.20.00124
37. Zheng, M., Hou, L., Ma, Y., Zhou, L., Wang, F., Cheng, B., Wang, W., Lu, B., Liu, P., Lu, W., & Lu, Y. (2019). Exosomal let-7d-3p and miR-30d-5p as diagnostic biomarkers for non-invasive screening of cervical cancer and its precursors. Molecular Cancer, 18(1). https://doi.org/10.1186/s12943-019-0999-x
38. Zhou, W., Huang, C., Zou, X., Lu, Y., Shen, C., Ding, X., Wang, H., Jiang, H., & Chu, Y. (2017). Exhaled breath online measurement for cervical cancer patients and healthy subjects by proton transfer reaction mass spectrometry. Analytical and Bioanalytical Chemistry, 409(23), 5603–5612. https://doi.org/10.1007/s00216-017-0498-0
About Renaldo Pool, BHSc
As a Medical Laboratory Scientist, I've developed a passion for scientific research and writing. I combine theory and practice to explore healthcare advancements. My lab expertise helps me investigate areas for improvement in healthcare through research and practical implementation. I aim to conduct thorough studies to advance medical knowledge and aid healthcare professionals in decision-making. Ultimately, I strive to bridge the gap between research and application for a positive impact in the healthcare profession.


